Latoplast
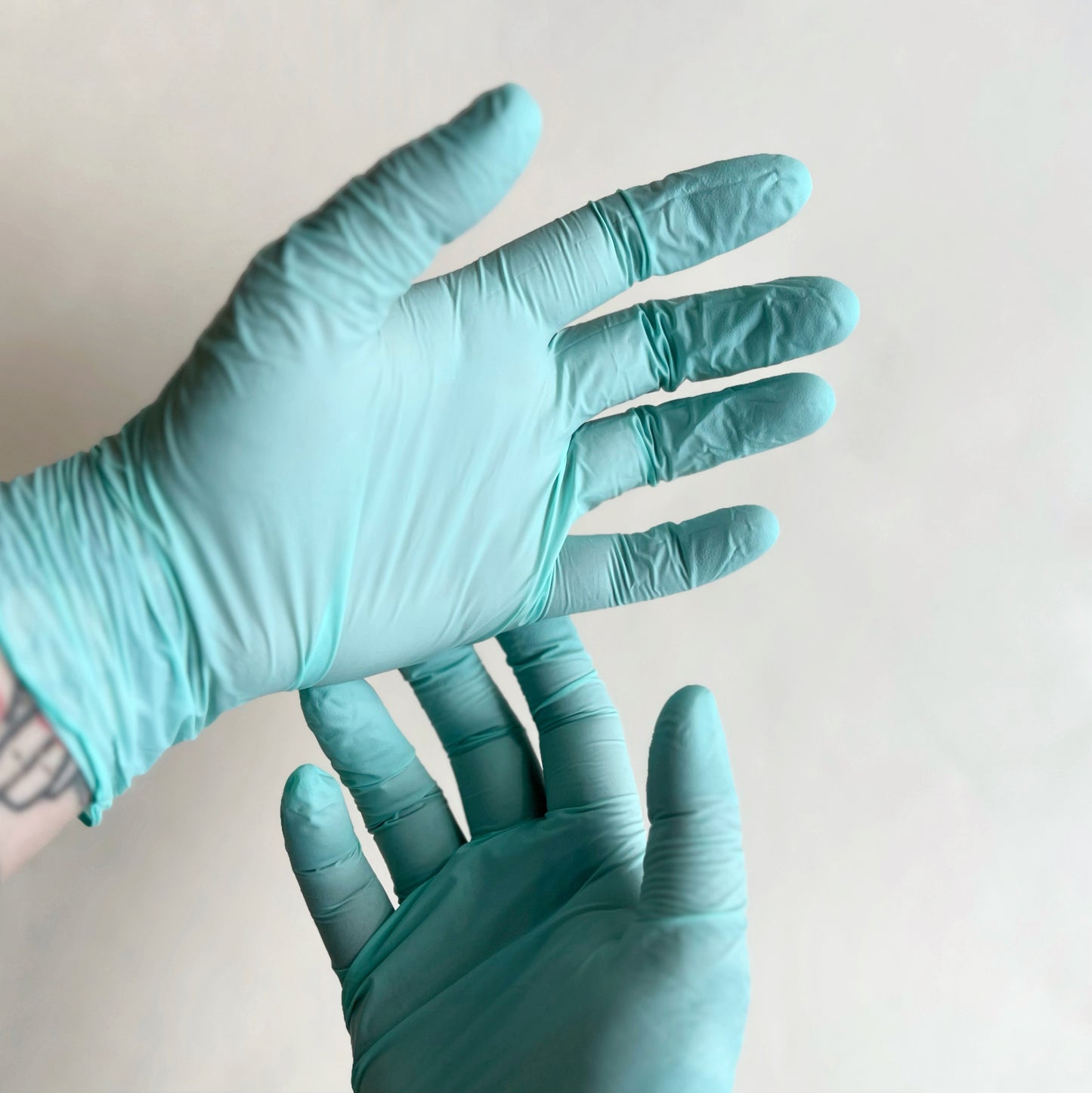
Mint Biodegradable Nitrile Gloves
Mint Biodegradable Nitrile Gloves
Couldn't load pickup availability
Description
Eco-responsible accelerator-free nitrile gloves that biodegrade within 3 - 5 years, without leaving behind harmful particles. They are Virus/Bacteria/Fungi protected and classified as a class 1 medical device.
Accelerator-free nitrile gloves are nitrile gloves that have no chemical accelerants used in the production process. They are made using the same raw material as regular nitrile gloves but with a slightly different formula without the accelerators. Because of this, they are better for the artist and the environment.
Features
- Animal derivative free
- Accelerator free
- 100% nitrile with organic compound additive
- 0.09mm / 3.6-mil thick
- 240mm / 9.5 inches long
- Zero natural rubber latex proteins
- Powder free
- Rolled cuff
- Sizes S to XL contain
Benefits
- Performs same as regular nitrile disposable gloves
- 100% biodegradable
- Decreased risk of allergies
- High performance of protection
- Second-skin fit & feel
Biodegradability
Our gloves are made of a mixture of nitrile and organic material that our manufacturer blends together which is why they perform the same way regular nitrile gloves do while still holding the capacity to degrade quickly.
The organic compound additive attracts micro-organisms (bacteria, fungi etc) that literally consume the glove material. Once this process is complete the only thing left behind is H20, CO2 and methane. Our manufacturing partners also conducted studies on how this process affects soil and plants that are exposed to this and found that it has no detrimental affects on their germination or growth patterns.
Certifications
Eco: ASTM D5511
Tested under high-solids anaerobic digestion conditions by ASTMD5511 (aka this is a true landfill test with the product surrounded by buried garbage).
Virus/Bacteria/Fungi: EN ISO 374-5:2016
Medical Device, Class 1, in accordance to MDR 2017/745
Quality: ISO 9001:2015, ISO 13485:2016 ISO 14001:2015
Other product related standards: EN 455-1:2020, EN 455-2015, EN 455-3:2015, EN 455-4:2009 EN 374-1:2016, EN 374-1:2016/A1:2018, EN 374-2:2019 EN 374-4:2019, EN 374-5 :2016. EN 16523-1:2015+A12018, EN420:2003+A1:2009
Share